What it shows:
The photoemission of electrons from a metal surface depends on the energy of the incident radiation and not on its intensity. Knowing the energy of the emitted photoelectrons and the frequency of the incident light, you can calculate a value for Planck's constant h.
How it works:
Using a mercury source, we have at our disposal three very bright visible lines, in the blue, green and yellow (doublet), and a rich selection of ultra-violet. Our main source is a Phillips Lifeguard 1000W street lamp with its outer (uv absorbing) glass casing removed. The audience is shielded from the source using lecture hall double doors and the light allowed to fall on a reflective diffraction grating across the hall; the spectrum is then imaged on a screen about 10m away (figure 1).
figure 1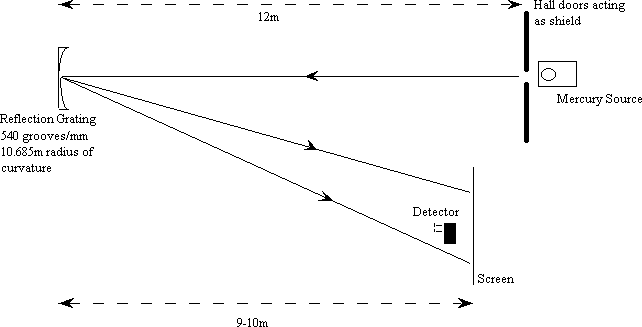
The detector is a PASCO Model AP-9368 h/e apparatus whose basic circuit is shown below. 1 Light of one color is incident on the cathode of a vacuum photodiode, which emits photoelectrons that then impinge on the anode. Because of the high input impedance of the amplifier, the charge continues to build until a stopping potential is attained. At this equilibrium value, the stopping potential can be read directly from the voltmeter V.
Move the detector so that one color falls onto the opening of the mask and also (moving the cover barrel aside) make sure the light strikes the photodiode window. Close the barrel, cover the opening with an opaque shield (such as the light block provided) and press the 'Push to Zero' button that discharges the anode. Remove the shield and record the potential difference when the voltmeter has stopped rising (about 10 sec.). Repeat for all the visible lines, and the strong UV which are easy to line up as both the mask and photodiode cover are phosphorescent.
figure 2. PASCO AP-9368 Simplified circuit diagram
The book values (A. Melissinos: Experiments in Modern Physics, Academic Press 1966) for the brightest lines and the stopping potentials we obtained are recorded in table 1.
table 1. Stopping potentials for the mercury lines
| Color | Frequency ν (×1014Hz) | Stopping Potential Vstop (V) |
| Yellow* | 5.2 | 0.79 ±0.05 |
| Green | 5.5 | 0.90 ±0.03 |
| Blue | 6.9 | 1.57 ±0.03 |
| UV1 | 7.5 | 1.80 ±0.03 |
| UV2 | 8.4 | 2.12 ±0.03 |
| UV3 | 8.9† | 2.33 ±0.03 |
*Yellow doublet stopping potentials could not be accurately distinguished
†We obtained this frequency (the highest bright UV line) from the graph
By plotting a graph of stopping potential against frequency, a straight line is yielded with slope = h/e. The above values give a slope of 3.9×10-15 resulting in h = 6.24×10-34Js. Work Function Φ = 2.1×10-19J ± 0.05×10-19J or 1.3eV
Setting it up:
The lamp and its transformer base can be mounted on an optics platform and placed outside the double lecture hall doors. The lamp takes about ten minutes to heat up. The crack between the doors should be just enough so that the lamp is fully visible to the grating. Place the reflection grating on an optics platform about 12m away and arrange so that it can see the lamp through the crack in the door. The reflected spectrum returns across the front of the hall to illuminate a projection screen 9.25m from the grating (the focal length of the mirror). Mount the detector on a camera tripod, and collect the light from right in front of the screen. The voltmeter is a Keithly digital multimeter set on 10V range, connected to the detector with a BNC cable (less wires to trip over); it will need a camera on its display. The UV line location can be found with a phosphor screen.
Comments:
Safety notes first. A lot of UV comes off the mercury lamp - 31 distinct UV lines - smell that ozone! Shielding the audience is important, and this is done by placing the source outside the lecture hall. The results from the PASCO device are amazingly accurate and reproducible, although the device is expensive and literally a black box. We'll include here some alternative equipment and setups.
We have a smaller 400W mercury source again with its glass envelope removed, mounted within a custom built elliptic housing with the lamp at one focus and the slit at the other. This housing, 45 × 40 × 24cm, is constructed of wood with a polished aluminum sheet reflector. Above the lamp is a ventilating lid. An adjustable slit controls the light output and line width (narrow slit means less light but you can resolve the yellow doublet). This lamp too can be used with the reflection grating, but also (if you don't mind losing the UV) a direct view (Amici) prism, as in figure 3. Focus the slit onto a white screen 8m away using a 5cm lens, then put the prism into place.
For a detector, we used an RCA 935 phototube with its glass envelope covered using black insulating tape. A window to the cathode was left exposed, but the anode had to be shielded from direct light also. The circuit (figure4) was custom built with the photodiode being held by a lab clamp on a transversely mounted optics platform so it could slide from one line to the next. The potentiometer is adjusted so that the current reading is zero; this is the stopping potential. Potential measurements are made using a Keithly electrometer on 10V scale. Current measurements are by Keithly digital multimeter on 1µA scale. The best results we obtained are recorded in table2.
figure 3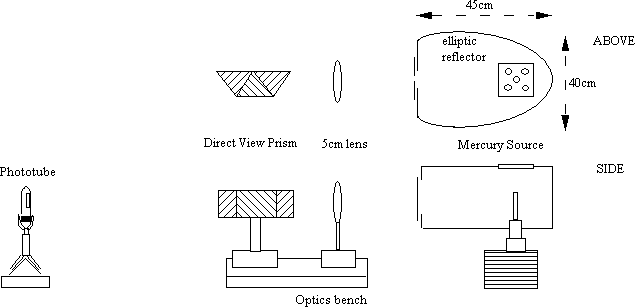
figure 4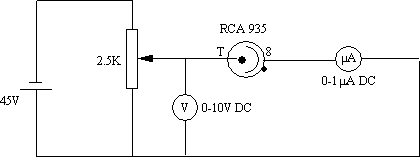
table 2
| Color | Frequency ν (×1014Hz) | Stopping Potential Vstop (V) |
| Yellow* | 5.2 | 0.509 ±0.01 |
| Green | 5.5 | 0.562 ±0.01 |
| Blue | 6.9 | 0.962 ±0.01 |
| UV1* | 7.5 | 1.051 ±0.01 |
| UV2* | 8.4 | 1.347 ±0.01 |
*UV lines obtained using reflection grating
Slope = 2.74×10-15 gives h = 4.39×10-34Js
Although this result is much less accurate than the PASCO setup, it has certain advantages. It is cheaper and requires less specialized equipment. It is also less of a black box; you can see the circuit and what each part of it does. It is more temperamental, so is perhaps more suitable for laboratory than lecture, but it does have a certain archaic style!
Further Reading:
Millikan, R. A., A Direct Determination of Planck's "h". Phys. Rev VII, 355-385 (1916)
Mellissinos, A. C., Experiments in Modern Physics (Academic Press, New York, 1966) pp.18-27
1 The apparatus is expensive and can be "homemade" if money is tighter than time. It seems that the PASCO device has been copied from a design published by D.W. Boys, M.E. Cox, and W. Mykolajenko, Am J Phys 46, 133-135 (1978), "Photoelectric effect revisited (or an inexpensive device to determine h/e)"