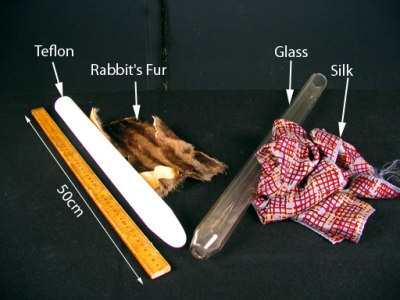
What it shows:
As long ago as 600 B.C., the Greek philosopher Thales knew that amber, when rubbed, would attract bits of paper and other light objects. Many other substances have this same property and can be electrified by rubbing. The kind of electrification (positive or negative) depends on the substances used.
How it works:
Many triboelectric series have been established but, although there is some agreement amongst them, most of these series are very dissimilar, even when the same materials are used. The following example is a typical series: 1
[rabbit's fur | Lucite | Bakelite | acetate | glass | quartz | mica | Nylon | wool | cat's fur | silk | paper | cotton | wood | amber | resins | metals | polystyrene | polyethylene | Teflon]
The materials at the beginning of the list are positive with respect to those at the end. Thus, to produce "resinous" electricity (DuFay's term), we figure you can't do much better than the [rabbit's fur | Teflon] combination and, indeed, they work extremely well. A Teflon rod (4cm dia x 50cm long) acquires a strong negative charge when rubbed with rabbit's fur 2 (much better than the traditional [cat's fur | resin] combination). For "vitreous" electrification, we rub a glass rod and silk together to produce a positive charge on the glass. These two materials are closer together in the series and consequently the charging effect is not as strong as the former. Acrylic (a.k.a. plexiglass) is even better than glass — stronger effect and more reliable. You can get negative charges by rubbing a cotton t-shirt on a PVC rod and positive charges by rubbing the same shirt on an acrylic rod.
The charging of the Teflon and/or glass can be shown by (1) attracting light objects such as Styrofoam puffs, 3 (2) touching it to an electroscope or, (3) holding it inside (not touching) the Faraday Ice Pail. The electroscope does not show the polarity of the charge but, by charging it first with the glass rod (positive) and then with the Teflon (negative), one can deduce that the two are oppositely charged by the fact that the Teflon first reduces (cancels) the charge on the electroscope before the electroscope becomes negatively charged. Alternatively, charge two separate pith balls with the Teflon and glass and show that the two balls attract or repel, depending on how you charged them (same or oppositely). If you choose to go the route of modern high-tech instrumentation, the Faraday Ice Pail hooked up to an electrometer 4 will tell you not only the polarity of the charge, but also the amount.
 Most of the electrostatic demonstrations that follow use an electrophorus for the supply of charge. Volta is generally credited with inventing the electrophorus perpetuum in 1775. Our modern version uses a sheet of Teflon for the dielectric plate which is charged by rubbing it with rabbit's fur. It was called perpetuum because the charge does not get used up (when charging by induction). A metal disk with an insulating handle is held over and close to the dielectric plate. The dielectric (negative in charge) repels the electrons from the lower surface of the disk to the top surface. These electrons are then allowed to escape by momentarily grounding the disk (by touching it with a finger). The disk with the remaining positive charge is then lifted away from the dielectric and this charge is used for the desired purpose. Any number of charges can be obtained from the electrophorous in this manner without depleting its charge. This is because work is done by the hand that lifts the disk against the attractive electrostatic force. The electrophorus will keep its charge for many months. Of course, if the metal disk is allowed to touch the dielectric, it will become charged by conduction (negatively) and this does deplete the charge of the electrophorus.
Most of the electrostatic demonstrations that follow use an electrophorus for the supply of charge. Volta is generally credited with inventing the electrophorus perpetuum in 1775. Our modern version uses a sheet of Teflon for the dielectric plate which is charged by rubbing it with rabbit's fur. It was called perpetuum because the charge does not get used up (when charging by induction). A metal disk with an insulating handle is held over and close to the dielectric plate. The dielectric (negative in charge) repels the electrons from the lower surface of the disk to the top surface. These electrons are then allowed to escape by momentarily grounding the disk (by touching it with a finger). The disk with the remaining positive charge is then lifted away from the dielectric and this charge is used for the desired purpose. Any number of charges can be obtained from the electrophorous in this manner without depleting its charge. This is because work is done by the hand that lifts the disk against the attractive electrostatic force. The electrophorus will keep its charge for many months. Of course, if the metal disk is allowed to touch the dielectric, it will become charged by conduction (negatively) and this does deplete the charge of the electrophorus.
Setting it up:
Humidity is the worst enemy of any electrostatic experiment. To minimize failures, keep all pieces of apparatus and paraphernalia in the heated closed cabinet. On particularly humid days (and there are lots in the summer), leave everything in the cabinet as long as possible and set the apparatus out on the bench in the hall just before lecture. Mount an infra-red lamp on the lecture bench and keep the fur, glass, Teflon, etc. bathed in the heat of the lamp. Keep a hair dryer handy on the really bad days.
A video camera with 25mm lens should be used to make the pith balls and electroscope visible to the class.
Comments:
How can one discuss electrostatics without this de rigueur demonstration?
1 From Electrostatics and its Applications, edited by A.D. Moore, (Wiley & Sons, NY, 1973). The author points out that it is certain that this series order will only be reproducible in rare instances. Conditions such as cleanliness and humidity affect it drastically. Even data on the same material is not reproducible owing to surface differences in manufacture and handling.
2 A cheap source of rabbit's fur is an old rabbit's fur coat. We bought our lifetime supply in a Thrift Store for $5.
3 Forget the traditional confetti paper - the Styrofoam puffs work better, are more visible, and today's packing material provides a never ending supply. Besides, you'll be helping the environment by recycling them for other purposes! Too bad you don't need very many. On the other hand, sometimes the Styrofoam puffs don't work very well. When all else fails, use dry downy feathers. They always work!
4 Keithley