What it shows:
β-rays emanating from a radioactive isotope are deflected from their straight line paths by a magnetic field.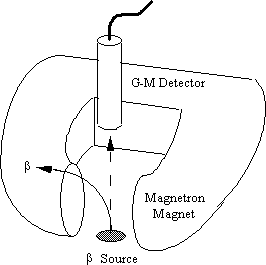
How it works:
90Sr/90Y, a "pure" beta-minus source, emits a continuous spectrum of fast electrons up to a maximum energy of 0.546/2.27 MeV. These electrons are detected by a Geiger counter and registered as audible "clicks." By interposing a strong magnetic field from a magnet, the electrons experience a Lorentz force which deflects them away from the detector. The audience hears the count rate go down (to background).
The experiment may be repeated with a gamma source, such as 137Cs or 60Co, to show that γ-rays are not affected by magnetic fields. Be aware, however, that these sources also emit beta rays which will screw up the experiment. A thin piece of plastic placed over the source will attenuate these unwanted rays (for 60Co, the maximum beta energy is 0.3MeV and thus a 0.5mm thickness should do the trick; 137Cs will require about twice this).
Setting it up:
Avoid using a steel cart as this distorts the magnetic field -- the wooden lecture bench top is best. With the beta source on the bench, the Geiger tube is supported by lab clamps/stand several centimeters above the source. The magnetron magnet, lying on its side, can then simply be slid into place during the demonstration.
Comments:
A clean, simple experiment and historically important. In 1899, Becquerel (and, independently, F. Giesel) found that, like cathode rays, part of the radiation emitted by uranium (the part called β-rays by Rutherford) could be deflected by a magnetic field, and in the same direction. Using Thomson's method, Becquerel measured the β mass/charge ratio and found it to be close to the electron's value, as measured by Thomson. It became clear that β-rays were just electrons, but with much higher energies than cathode ray electrons. 1
1 Steven Weinberg, The Discovery of Subatomic Particles, (Scientific American Library, NY, 1983).