Compression of gas within bicycle pump heats gas; alternatively, syringe PV=nRT (w/ Mac TC read-out).
What it shows:
An adiabatic process is one where no heat enters or leaves a system. Here we compress a gas adiabatically inside a bicycle pump. The work done on the gas increases its internal energy, so increasing its temperature in accordance with the first law of thermodynamics.
Increase in internal energy dU = dW the work done on the system
How it works:
Instead of allowing the air out of a bicycle pump we've mounted a thermistor 1 in the valve (figure 1a). When you pump, the air heats up, causing a decrease in resistance of the thermistor (it has a negative temperature coefficient). The temperature change is read as a voltage change in a simple circuit (see figure 1b). To get a positive voltage change for a positive temperature change, the voltmeter is connected across a series resistor.
figure 1a. detail of pump valve 1b. circuit diagram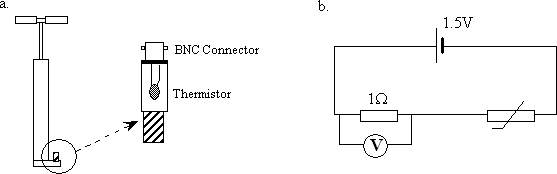
Setting it up:
The 1.5V cell and 1Ω resistor are mounted on a board. The pump itself has to sit on the floor, so you'll need long leads as everything else ought to sit on the bench. The voltmeter is a Keithly multimeter 2 set on a mV scale. You will get a voltage change in the range of 0.5mV for a couple of pumps. The multimeter display will need a camera on it.
Comments:
The indirect evidence of temperature rise by reading off a voltage rise isn't very satisfactory. It's a clear effect, but this demo needs redesigning to incorporate a direct temperature display. Rating *
1 Type GB32L2 Fenwell Electronics, Framingham Mass.
2 Keithley 160 digital multimeter. Keithley Instruments, Cleveland, Ohio.