What it shows:
Pure water is an electrical insulator. But provide an ionic compound in the form of salt, and you complete the circuit.
How it works:
A simple circuit with the mains supply connected to a 15W light bulb and two copper sheet electrodes (figure 1). The electrodes are placed in a 1500ml beaker containing distilled water. Distilled water is a very good insulator, with an autoionisation of 1:10-7 (the proportion of molecules in H3O+ + OH- form) it has a resistance of 20MΩcm-1. Adding an ionic compound in the form of common salt Na+ Cl- provides a huge number of extra charge carriers (2g ≈ 4×1022 ions) and turns the water into a conducting medium. The bulb lights!
figure 1. The apparatus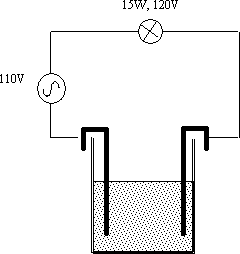
Setting it up:
The circuit uses a dedicated cable with a mains plug at one end and clip leads at the other. The best way to hold the electrodes in place is to fold the copper strip over the beaker rim. Only a small amount of salt is needed; a shaker or packet from the nearest cafe will suffice.
Comments:
The demo uses the 110V mains supply, so ensure the user is aware of the high voltage (provide a note or a "High Voltage" sign). It is a good demo for discussing electrical safety, and why electrical appliances should be kept away from your distinctly non-pure bath water.